Scaling up the use of the First Malaria vaccine in the context of the COVID19 pandemic, experiences from Ghana, Kenya and Malawi- The RTS,S/AS01 malaria vaccine
by Gideon Kwame Sarkodie OSEI, HJN Member in Ghana

In October 2021, the World Health Organization (WHO) recommended expanded use of RTS,S/AS01, the first malaria vaccine, among children living in regions with moderate to high malaria transmission. If implemented broadly, the vaccine could save tens of thousands of lives each year.
The WHO recommendation for wide use of the malaria vaccine is informed by findings from the ongoing pilot rollout of the vaccine in Malawi, Ghana, and Kenya, and by other RTS,S evidence. Vaccine implementation has been led by the Ministry of Health in Ghana.
Malaria vaccine: an example of innovation at work
The malaria vaccine RTS,S, is an example of innovation at work and a scientific breakthrough. It is the first vaccine recommended for use against a human parasitic disease of any kind. The vaccine is a major step forward for malaria control, child survival, and health equity. The vaccine substantially reduces malaria illness and death. This new tool is needed now more than ever. In 2020, nearly half a million African children died from malaria. This comes down to 1 child dying of malaria every minute.
Progress of the malaria vaccine pilots
Since 2019, more than 1 million children have been reached with at least one dose of malaria vaccine through pilot introductions in Malawi, Ghana, and Kenya. These numbers indicate strong community demand and the capacity of childhood vaccination platforms to effectively deliver the vaccine to children. In Ghana, about 36,000 of children have received at least one dose of the vaccine with an uptake around 60 percent.
Key findings: Feasibility
According to Mr. John Bawa, Africa Lead for Vaccine Implementation – PATH’s Center for Vaccine Innovation and Access – it is operationally feasible to provide the malaria vaccine to the target age-group through routine immunization programs.
“Good coverage reached with first 3 doses through routine systems. Introduction did not negatively impact uptake of other vaccines, insecticide-treated bed nets (ITNs), or care-seeking behavior. The vaccine was able to reach children not yet protected by ITNs, extending the reach of malaria preventive measures to vulnerable children. 2/3 of children not sleeping under an ITN receiving the RTS,S/AS01 vaccine . Overall, more than 90% of children benefitted from either sleeping under an ITN or RTS,S/AS01 vaccination,” says Bawa.
Key findings: Safety and impact
On safety, Mr. John Bawa said the vaccine has a favorable safety profile, “no evidence in the pilot evaluations that the safety signals seen in the Phase 3 trial were related to the RTS,S/AS01 vaccine. No new safety concerns after over 1 million doses provided.“
On the impact, he added that, “vaccine introduction resulted in a substantial and statistically significant reduction in hospitalized severe malaria and hospitalization with malaria infection .Among children age-eligible for vaccination, when introduced in the real-world setting. The impact seen even in setting with good ITN use and access to ACTs, confirms vaccine can have substantial added benefit to reduce child illness and death from malaria.”
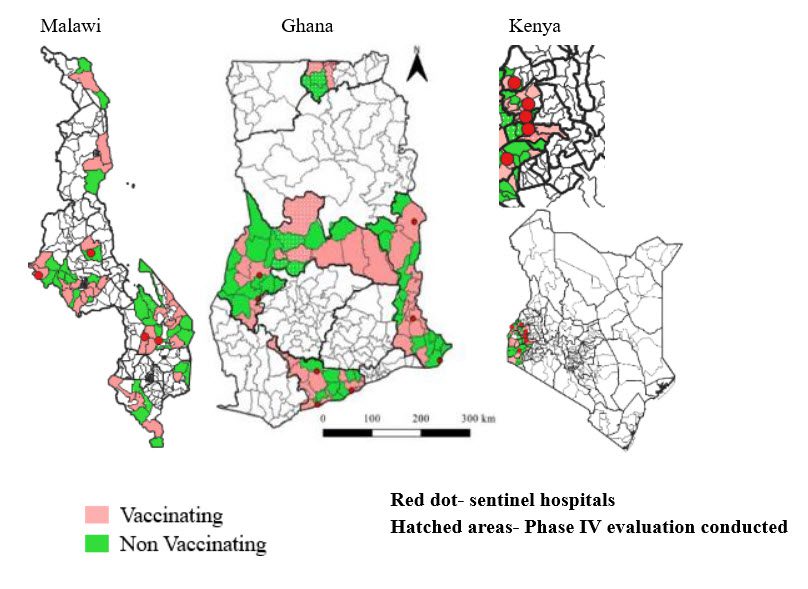
Country perspectives: Ghana, Kenya, and Malawi – Areas selected for Malaria Vaccine Implementation Program (MVIP) implementation
Specific areas (regions/counties) were selected in Ghana, Kenya, and Malawi through a rigorous randomization process.
Lessons learned from the introduction of malaria vaccines
So many lessons have been learnt from Malawi, Ghana, and Kenya since the malaria vaccines were first introduced. Key amongst these is the importance of coordinating with national malaria programs. This is especially critical since the vaccines complement other interventions. Community acceptance is high because people are aware of malaria and its impact. Regular and supportive supervision has shown to identify and resolve challenges during implementation. Community health workers play a critical role in creating and sustaining demand for vaccines. Lastly, once the malaria vaccines are introduced, sustained communication and stakeholder engagement is necessary.
First Malaria Vaccine: key success areas
- High-level political support and commitment
- Robust crisis communication strategy
- Strong collaboration with community and stakeholders
- Concurrent evaluations (costing, safety, mortality, household, health utilization survey)
- Good inter-sectoral collaboration
- Establishment of a Joint Malaria Vaccine Safety Committee by FDA
- Sustained performance and uptake during COVID-19 and stock-outs
- Close to 80% of districts achieving at least 60% coverage (for a new vaccine)
- Maintaining drop-out rates between doses 1 and 3 (< 10%)
What this means for malaria control
Improved equity in accessing malaria prevention interventions will offer an opportunity for stronger program integration for the delivery of child health interventions (EPI and NMCP). There will also be the ability to leverage the malaria program experience to tailor sub-national malaria interventions.
Next steps
The next steps for the first malaria vaccine are well underway. Countries have begun considering whether to adopt the vaccine as part of their comprehensive malaria control plans.
PATH, an international global health research NGO, says a grant of close to US$ 5 million will enable expanded use of the RTS,S vaccine in pilot areas that have not yet received the vaccine. The award will provide each pilot country with funds to expand malaria vaccination in the pilot areas through the end of 2023, the formal end of the pilot program. The grant to PATH was recommended by GiveWell, a US-based non-profit, for funding by Open Philanthropy, also based in the United States.
The availability of funds will allow the pilot countries to begin expanding use of the vaccine in the pilot areas before the end of 2022, prior to GAVI funding becoming available.
Countries eligible for GAVI support that decide to adopt the vaccine—including the pilot countries—will need to apply to GAVI, the Vaccine Alliance; the first application deadline is expected in September 2022. Initial funding of close to $160 million has been secured from GAVI to support the introduction, procurement and delivery of the malaria vaccine for GAVI-eligible countries in sub-Saharan Africa. In addition, WHO guidance is now available to countries as they consider whether and how to adopt the vaccine as an additional tool to reduce child illness and deaths from malaria.
WHO has published an updated position paper on the malaria vaccine, and WHO’s recommendation for the vaccine was recently added to the WHO guidelines for malaria.
Expanding vaccination in Ghana: where we are and next steps
This expansion is expected to start in Q4 this year, depending on Q3 preparations. The next stage is for the country to apply for Gavi support for commercial doses. National Coordinator for Ghana Malaria Control Programme, Dr Keziah Malm says Ghana needs renewed commitment in the fight against malaria. She made the call during the commemoration of this year’s World Malaria Day in Ghana. Dr Malm noted that malaria is the highest disease expenditure under the National Health Insurance Scheme and it reduces Ghana’s GDP by 0.25 to as high as 6 percent.
“It is still the number one cause of outpatient attendance in our facilities and affects millions of people,” said Dr. Malm.
She noted that the current National Malaria Strategic plan which spans 2021 to 2025 has three main goals; which are to reduce malaria mortality by 90 per cent, to reduce malaria case incidence by 50 per cent and to achieve malaria pre-elimination in at least 6 districts by 2025 using 2019 as a baseline.
Partners are working to increase vaccine supply
Anticipated high demand for the vaccine will far outstrip supply in the next few years. WHO and partners are working hard to accelerate increased supply, through increased manufacturing capacity of RTS,S and by facilitating the development of other first-generation and next-generation malaria vaccines.
Intention of the Malaria Vaccine Allocation Framework
The Framework aims to offer guidance globally on the allocation of malaria vaccines between countries, and to offer high-level guidance nationally on the prioritization of areas for vaccination within countries (while respecting sovereign decision-making)while supply is limited. Once finalized, all relevant stakeholders will adhere to the Framework, including malaria endemic countries, Gavi in its application guidance and processes for providing support; UNICEF in its procurement; manufacturer(s) and other partners as they consider their financial and technical support. The Framework will be designed in a way that allows prioritization of any level of supply that may become available overtime (until supply fully meets demand).
PATH’s role: Access and supply
PATH is actively engaged with WHO, GSK, GAVI, UNICEF, and other stakeholders toward ensuring equitable access to malaria vaccines and future demand-supply balance, in the context of a healthy malaria vaccine market.
PATH is working in partnership with stakeholders, vaccine developers and manufacturers, to ensure sustainable, long-term supply of malaria vaccines. An agreement for product transfer was signed among PATH, GSK and Bharat Biotech (BBIL) in December 2020. PATH is helping to lead the knowledge transfer of access related activities from GSK to BBIL. PATH has approximately 2.5 million donation doses for use to further optimize vaccine impact. The organization is currently exploring options for use of these doses with key stakeholders. They are also helping to identify further investments required to increase impact of malaria vaccines. This includes seeking funding for manufacturing capacity for both antigen and adjuvant components.